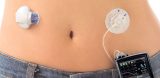
In September of 2016, a major breakthrough developed in the treatment of Type 1 Diabetes. The first artificial pancreas, known as the Medtronic’s MiniMed 670G System, was approved by the FDA. This hybrid, closed loop system, includes a glucose meter, a continuous glucose monitor (CGM), and an insulin pump. The first component, a glucose meter, is used to calibrate the CGM. The second component, the CGM, evaluates blood glucose throughout the day. The last component includes a motorized infusion pump that delivers either insulin or glucagon. However, Medtronic’s MiniMed 670G System still relies on diabetics to closely count carbohydrates in their food and then enter these amounts into their system.
A fully automated, closed loop system, such as iLet, senses rising glucose levels, particularly at mealtimes, and adjusts insulinautomatically (Diabetes Week, 2017). This automated medical device eliminates the carbohydrate counting process for diabetics. Although it has not been FDA approved, it is currently being tested in various trials funded by the National Institute of Health (NIH) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). This CGM and pump system consists of an insulin-only system, as well as a system that uses two hormones–glucagon and insulin–within the pump system, in conjunction with the CGM (Appold, 2017).
The CGM is constantly collecting blood sugar values in order to achieve better control. These values determine whether or not the pump should administer insulin to decrease glucose values, or administer glucagon to increase glucose values.
The approximate annual cost of treatment for both pre-diabetes and diabetes in the United States is an astonishing $322 billion (Appold, 2017). However, major strides in the technological development and treatment of diabetes can indirectly help to reduce overall treatment costs. NIH research found that automatic basil insulin delivery, can help reduce diabetes complications, including nerve, eye, and kidney diseases. This delivery system helps to tightly regulate and control blood sugar levels, decreasing the amount of hospital visits, and therefore reducing overall treatment costs.
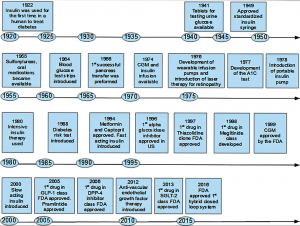
Below is a timeline of events that are historic to the technological advancements and treatment of diabetes. To see an extensive version of this timeline, please visit http://www.diabetes.org/about-us/75th-anniversary/timeline.html?loc=75.
References
75th Anniversary Timeline. (n.d.). Retrieved July 24, 2017, from http://www.diabetes.org/about-us/75th-anniversary/timeline.html?loc=75
Appold, K. (2017). Diabetes improvements: Innovations shake up the industry. North Olmsted: Advanstar Communications, Inc.
Beta Bionics | Introducing the iLet. (n.d.). Retrieved July 24, 2017, from https://www.betabionics.org/
Center for Devices and Radiological Health. (n.d.). Recently-Approved Devices – The 670G System – P160017. Retrieved July 24, 2017, from http://wayback.archiveit.org/7993/20170111141252/http:/www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm522764.htm
Iturralde, E., Tanenbaum, M. L., Hanes, S. J., Suttiratana, S. C., Ambrosino, J. M., Ly, T. T., . . . Hood, K. K. (2017). Expectations and attitudes of individuals with type 1 diabetes after using a hybrid closed loop system. The Diabetes Educator, 43(2), 223-232. doi:10.1177/0145721717697244
Nutritional and metabolic diseases and conditions – type 1 diabetes mellitus; artificial pancreas benefits young children, trial shows. (2017). Diabetes Week
Nutritional and metabolic diseases and conditions – type 1 diabetes mellitus; four pivotal NIH-funded artificial pancreas research efforts begin. (2017). Diabetes Week
Written by Nicole Lindel ~ Nutrition Education Master’s Student at Columbia University
